Australia approves new drug to treat early Alzheimer’s disease | Alzheimer’s
Australian drug organizer has agreed to a new drug to treat the early stages of Alzheimer’s disease, but experts warn that less than one in five people suffer from dementia will be eligible for treatment that may cost more than $ 80,000 of his pocket.
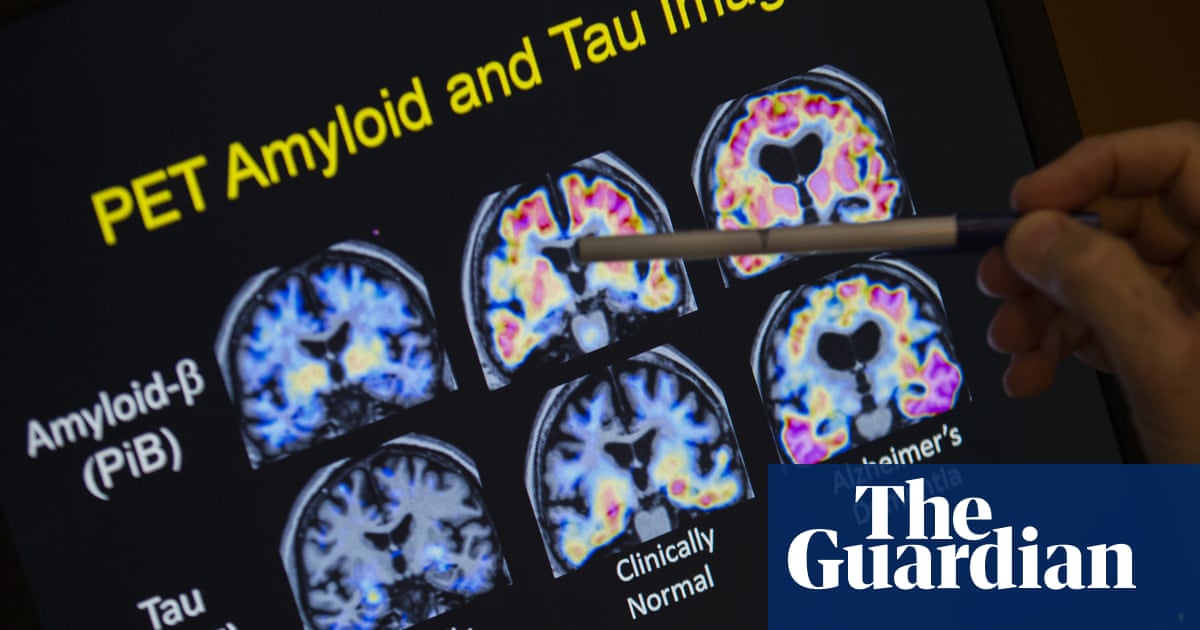
TGA DONNEMAB, which was sold under the name of the Kisunla brand and developed by the ALI Lilly pharmaceutical company, was registered to treat adults with early Alzheimer’s disease and who have a specific genetic image.
Donnemab is given as an intravenous leakage across the arm every four weeks for a maximum of 18 months, and it works by targeting amyloid proteins in the brain – where researchers believe that it contributes to Alzheimer’s disease.
Professor Christopher Row, director of the Australian dementia network, said that the registration represents the first new treatment for Alzheimer’s disease in 25 years, and the first time that treatment has affected the results of the disease.
Row said that the drugs that were approved 20 years ago are temporarily improved, “but this drug actually slows down the rate of decrease by about a third.”
While this is “an important first step” in the hope of using a group of medications in the end to stop the development of the disease, Row has warned that expectations may be somewhat high because most people with Alzheimer’s disease will not be already qualified for this.
He said that the person is qualified for the drug, the person must be in the early stages of Alzheimer’s disease, the stage of moderate cognitive weakness, and is not subject to the factors of the danger of the drug.
“The biggest obstacle is the diagnosis of people who are diagnosed after it is too late if they are already outside the early stage of Alzheimer’s disease.”
The drug has also been associated with the side effects of brain swelling and bleeding, which occurs in a much higher occurrence in people who carry the APOE ε4.
TGA registration excludes people with genes, which Row said is an “unfortunate situation” because the genetic alternative itself raises the risk of developing the younger, often in the 1960s.
Before starting treatment, patients must be tested to determine their genetic condition and have magnetic resonance imaging checks to detect any preliminary signs of brain bleeding and swelling that may make it unsafe take of the medicine. Patients also need to receive continuous MRI control for any brain bleeding during treatment.
Row said: “We actually appreciate that only 10 to 20 % of people with dementia will be suitable for the drug, but this is still a large number due to the presence of 400,000 people in Australia with dementia, and perhaps 40,000 diagnoses every year.”
To qualify for treatment, patients will also have to confirm the diagnosis of early Alzheimer’s disease through a cotton hole or amyloid PET, which can confirm the presence of amyloid proteins – not covered by medicare. The drug is not on the pharmaceutical interest scheme (PBS).
“This treatment will cost $ 80,000, and the individual will currently have to pay from his pocket. The drug itself may be about $ 40,000, but then there are specialized fees, leakage fees, magnetic resonance imaging survey, and amyloid wiping for pets for payment against.”
After promoting the newsletter
Eli Lilly applied to include Kisunla on PBS, which will be reviewed by the Pharmaceutical Advisors Consulting Committee (PBAC) in July.
“There is still a fair way to go, to determine whether the federal government will be offered to be if that, and if so, that is, the patients,” said the Minister of Health, Mark Beler, on Thursday.
“You will also need to consider the associated survey that must occur to patients.
“A little way to go, but very exciting news for Alzheimer’s community,” said Bater.
There was Controversy between researchers Whether slowing the rate of the decrease by a third is important enough to justify the risks of this group of drugs.
“The patient and his family must be completely informed that they must make this decision. It is likely that he will buy them for an additional two years before they reach the intense dementia stage, and they must decide whether the money and risks deserve it,” Row said.
“With dementia, the second main cause of the death of the Australians, and the main cause of the death of Australian women, we welcome any steps heading towards improving the lives of people who live with dementia and their families and care providers,” said Professor Tania Boucanan, CEO of Dementia Australia.
The Australian dementia network conducts an experiment to enable GPS to perform blood tests to confirm Alzheimer’s disease, which will help build the ability of doctors who can diagnose people and allow people to get treatment sooner.



