Our lifesaving device for small babies is stuck in development | Medical research
We have also found “outside the box” about Alexander Master’s long reading (Several life -saving drugs fail due to lack of financing. But there is a solution: the desperate rich, March 11Interesting. We agree with the views of Professor Roger Bayston on problems in obtaining innovative devices in commercial production, through organizational obstacles, and clinical use (Messages, March 17).
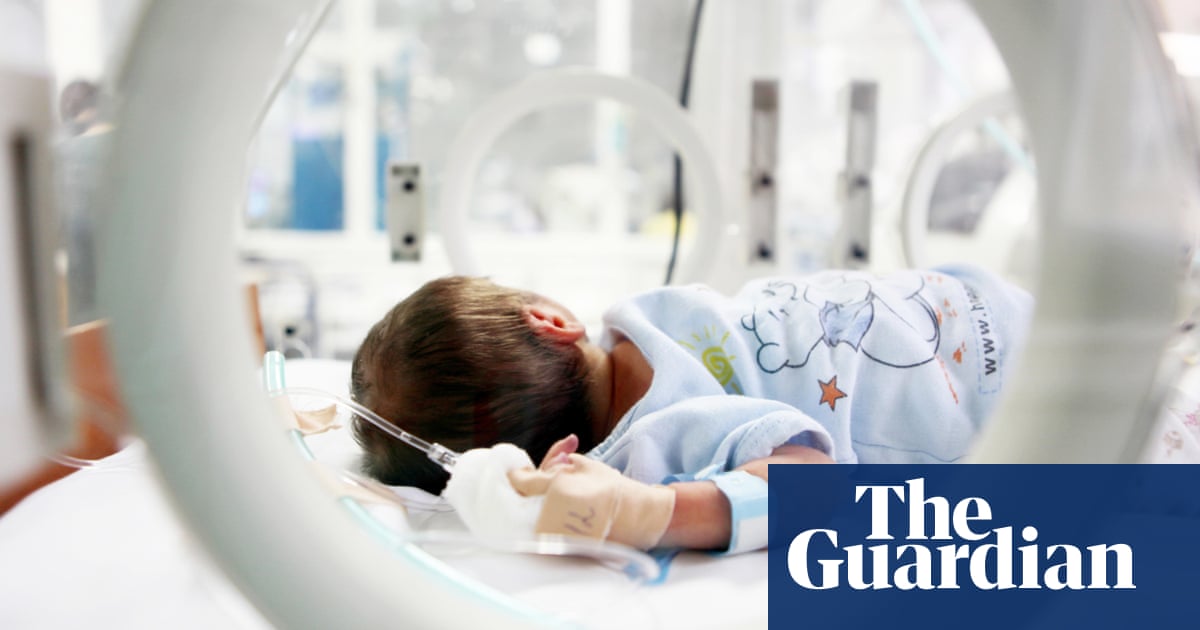
These problems are exacerbated when the new device aims to treat a rare disease or a small sub -section of a more common problem, in our case the developing a dialysis device to treat young children. This device, which is the Newcastle dialysis system and the NIDUS (NIDUS) system, was invented in response to parental pressure for “doing something” when newborns who undergo a large surgery (often for congenital anomalies such as abdomen or heart disease) undergo failure in the kidneys and need the kidneys to keep them alive and provide time to recover.
This is not fulfilling the clinical need for children who need dialysis but it cannot be treated with peritoneal dialysis, and in the UK there are no dialysis devices to do so safely. Consequently, in the face of any alternatives, designed and licensed machines are used only for adults and children over the age of 8 kg, despite the warnings of manufacturers and possible serious problems known to do so. There is no record for the devices used outside the license and any problems that arise.
The new NIDUS has been developed for 20 years of handicrafts through various preliminary models to the current device, with time, experience and skills in NHS medical engineering, nursing and medical employees. She underwent a successful clinical trial funded by the National Institute of health And care research (i.e. taxpayer). However, it is now stuck, and it lacks experience and relevant money to obtain organizational approvals. No one will make money from this device, and manufacturing companies are limited as they can spend to help develop.
In the United Kingdom, while there are paths for “orphan” drugs used in rare diseases, there are no “orphaned devices like this. It is clear that we need organizational systems for patient safety, but with tracks of penetration technology.
Dr. Heather Lambert and Dr. Malcolm Kultidd
retired pediatrics Kidney scientists, Newcastle on Tine




